Head to Head: the ethics of vaccine passports and COVID passes
Crosspost with the Conversation, read the original article here
by Alberto Giubilini (University of Oxford) and Helen Kennedy (University of Sheffield)
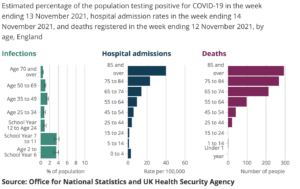
COVID passes for England were given the green light in parliament in December, with 369 MPs voting in favour and 128 against. From now on, people attending large events will be required to show proof of vaccination – two doses, to become three after a “reasonable” amount of time – or a recent negative lateral flow test. The schemes were already being used in other parts of the UK, with slight differences.
First mandated in Israel, COVID passports consist of a paper or digital document that provides proof you have been fully vaccinated against COVID, have recovered from the virus, or have recently tested negative. The vaccination certificates were adopted widely around Europe, for sole domestic use in some countries and as travel passes in others.
But some critics have questioned the need of enforcing a passport, on the basis that while vaccines have been proven to reduce the chance of falling seriously ill, they do not fully stop the spread of the disease. And an increasing number of commentators have leveraged ethical arguments, comparing vaccine passports to a form of state coercion. We asked digital society professor Helen Kennedy and ethics researcher Alberto Giubilini for their views.
Alberto Giubilini: We’ve never had to deal with a pandemic like this in our lifetime. For the last year and a half, we’ve undertaken all sorts of big experiments, some of which have yet to be proven to work. One is lockdowns: the huge costs involved hadn’t been predicted or taken into consideration, even when introduced for the second or third time.Read More »Head to Head: the ethics of vaccine passports and COVID passes